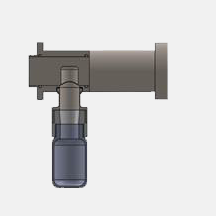
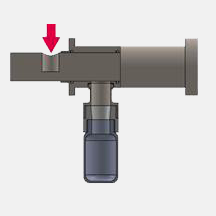
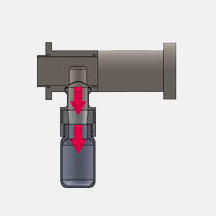
The Intrusive Oyster TM Samplemaster is designed for applications where it is required that the piston moves into the host vessel, and actively collects the sample which it then transports out of the vessel/pipe and deposits into the sample collector.
All OysterTM branded sanitary valves are designed according to cGMP standards, for trouble free operation and easy maintenance. The valve design ensures optimal hygienic and functional characteristics and eliminates recesses with hard-to-clean corners. OysterTM branded valves are manufactured to exacting tolerances and finished to the highest pharmaceutical standards.
![]()
FEATURES
| CE Certification | ATEX: The Oyster Samplemaster Valve is outside the scope of the ATEX Directive 94/9/CE (article 1, paragraph 3a), does not require the relative marking and is not subject to the procedure of conformity specified by the ATEX directive 94/9/CE. The Oyster Samplemaster Valve may be used in hazardous areas at risk of explosion classified as zone 1/21 PED:according to the requirements of Article 3 paragraph 3 of the PED the valve is constructed following Sound Engineering Practice and is suitable for fluids of Group 1. |
| Diameter | DN 25 / 50 / 80 – (1”-2”-3”) |
| WIP | CIP/WIP suitable |
| Operation | Hand gear wheel / other automation options |
SPECIFICATIONS
| M.O.C. CONTACT PARTS | AISI 316L, 22, (other alloys available upon request) |
| M.O.C. OTHER PARTS | AISI 304 |
| M.O.C. SEALS (all FDA 177.2600 compliant) | FEP, FFKM |
| AVAILABLE CONNECTIONS | TC BS 4825-3, TC ASME/BPE, TC DIN32676, Weld End, Flange |